Cards In This Set
| Front | Back |
|
Linear Triatomic
|
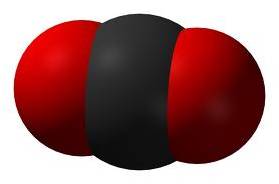 2 double bonds no non-bonding pairs on central atom |
|
Tetrahedral
|
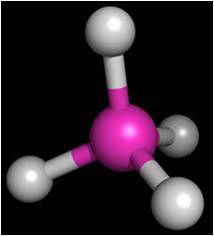 4 single bonds no non-bonding pairs on central atom |
|
Trigonal Planar
|
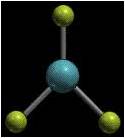 3 single bonds and no non-bonding pairs OR 1 double bond and 2 single bond with no non-bonding pairs |
|
Pyramidal
|
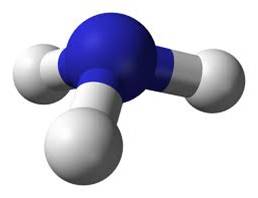 3 single bonds 1 non-bonding pair off central atom |
|
Linear
|
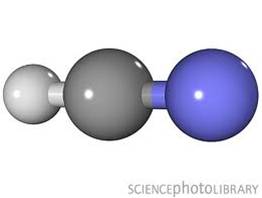 1 triple and 1 single bond non non-bonding pairs off central atom |
|
Bent Triatomic
|
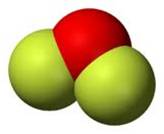 2 single bonds 2 non-bonding pairs off central atom |
|
Diatomic Linear
|
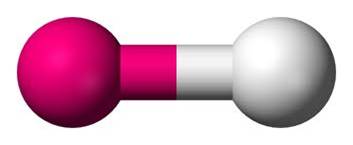 1 single bond and 3 non-bonding pairs OR 1 double bond and 2 non-bonding pairs OR 1 triple bond and 1 non-bonding pair |



