Cards In This Set
| Front | Back |
  |
example: CO2
total bonds + e pairs: 2
lone e pairs: 0
geometry: linear
shape: linear
bong angles: 180
hybrid orbitals: sp
|
  |
example: CH2O
total bonds + e pairs: 3
lone e pairs: 0 geometry: trigonal planar shape: trigonal planar bond angles: 120 hybrid orbitals: sp2 |
  |
example: SO2
total bonds + e pairs: 3
lone e pairs: 1
geometry: trigonal planar
shape: v-shape or bent
bond angles: 120
hybrid orbitals: sp2
|
  |
example: CH4
total bonds + e pairs: 4
lone e pairs: 0
geometry: tetrahedral
shape: tetrahedral
bond angles: 109.5
hybrid orbitals: sp3
|
  |
example: NH3
total bonds + e pairs: 4
lone e pairs: 1
geometry: tetrahedral
shape: trigonal pyramid
bond angles: 107
hybrid orbitals: sp3
|
  |
example: H2O
total bonds + e pairs: 4
lone e pairs: 2
geometry: tetrahedral
shape: v-shape or bent
bond angles: 104.5
hybrid orbitals: sp3
|
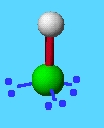 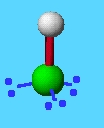 |
example: HCl
total bonds + e pairs: 4
lone e pairs: 2
geometry: tetrahedral
shape: linear
bond angles: 180
hybrid orbitals: sp3
|
  |
example: PCl5
total bonds + e pairs: 5
lone e pairs: 0
geometry: trigonal bipyramid
shape: trigonal bipyramid
bond angles: 120, 90, 180
hybrid orbitals: sp3d
|
  |
example: SF4
total bonds + e pairs 5:
lone e pairs: 1
geometry: trigonal bipyramid
shape: see-saw
bond angles: 120, 90, 180
hybrid orbitals: sp3d
|
  |
example: ClF3
total bonds + e pairs: 5
lone e pairs: 2
geometry: trigonal bipyramid
shape: t-shape
bond angles: 90, 180
hybrid orbitals: sp3d
|
  |
example: KrF2
total bonds + e pairs: 5
lone e pairs: 3
geometry: trigonal bipyramid
shape: linear
bond angles: 180
hybrid orbitals: sp3d
|
  |
example: SF6
total bonds + e pairs: 6
lone e pairs: 0
geometry: octahedral
shape: octahedral
bond angles: 90, 180
hybrid orbitals: sp3d2
|
  |
example: ICl5
total bonds + e pairs: 6
lone e pairs: 1
geometry: octahedral
shape: square pyramid
bond angles: 90, 180
hybrid orbitals: sp3d2
|
  |
example: XeF4
total bonds + e pairs: 6
lone e pairs: 2
geometry: octahedral
shape: square planar
bond angles: 90, 180
hybrid orbitals: sp3d2
|



