Cards In This Set
| Front | Back |
 Name This |
Electron Arrangement: LinearMolecular shape: Linear 2 Bonding Groups180 Degrees
|
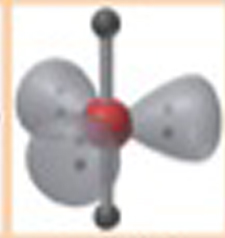 Name This |
Electron Arrangement: Trigonal BipyridimalMolecular Shape: Linear2 bonding groups180 Degrees
|
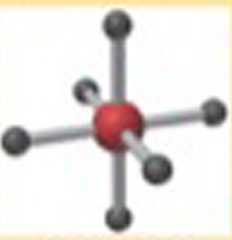 Name This |
Electron Arrangement: OctahedralMolecular Shape: Octahedral6 bonding groups90 degrees
|
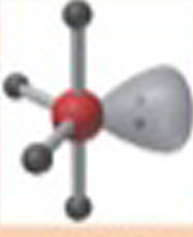 Name This |
Electron arrangement: Trigonal BypyridmialMolecular shape: seesaw4 bonding groups<90 degrees<120 degrees
|
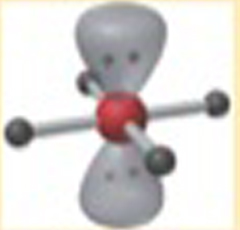 Name This |
Electron arrangement: Octahedralmolecular shape: square planar 4 bonding groups90 degrees
|
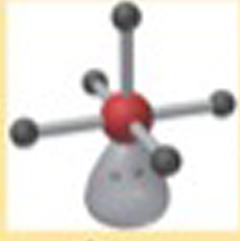 Name This |
Electron arrangement: Octahedralmolecular shape: square pyradmial 5 bonding groups<90 degrees
|
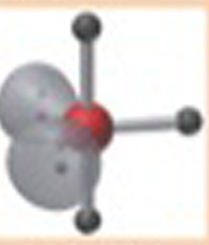 Name This |
Electron arrangement: Trigonal Bipyradmidalmolecular shape: T-Shaped3 bonding pairs<90 degrees
|
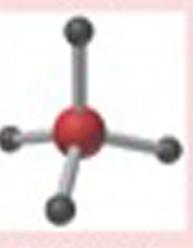 Name This |
Electron arrangement: Tetrahedralmolecular shape: Tetrahedral4 bonding pairs109.5 degrees
|
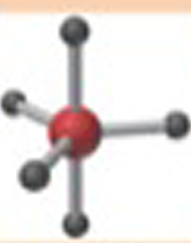 Name This |
Electron arrangement: Trigonal Bipyramidal molecular shape: Trigonal Bipyramidal 5 bonding pairs90 degrees120 degrees
|
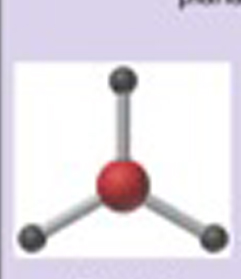 Name This |
Electron arrangement: Trigonal Planarmolecular shape: Trigonal Planar 3 bonding pairs120 degrees
|
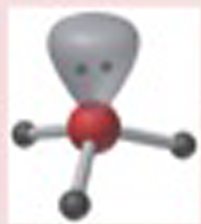 Name This |
Electron arrangement: Tetrahedralmolecular shape: Trigonal Pyramidal3 bonding pairs<109.5 degrees
|
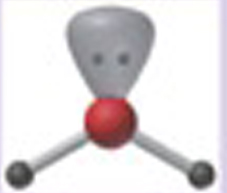 Name This |
Electron arrangement: Trigonal Planarmolecular shape: V-Shaped or Bent2 bonding pairs<120 degrees
|
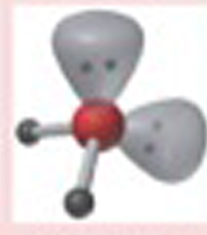 Name This |
Electron arrangement: Tetrahedralmolecular shape: V-Shaped or Bent2 bonding pairs<109.5 degrees
|



