Cards In This Set
| Front | Back |
|
Micelle charge
|
Generally have an over-all negative charge on their flat surfaces. this electrostatic charge attracts and holds on to ions in the soil solution. positive charges may occur along broken edges of clay layers. negative charge attracts positive charged ions in soil solution
|
|
Isomorphous Substitution
|
This is the process that gives clay its electrostatic charge. during clay mineral formation, a different atom may get wedged in the center of a forming tetrahedron or octahedron - instead of the usual silica or aluminum. this causes an imbalance in valance.
|
|
Isomorphous substitution charge change
|
A cation is replaced by another cation with a less positive charge resulting in an extra or unbalanced negative wharge. O2 is normally -2 each, but share with other tetrahedron so have Si+4 surrounded by -4 = neutral. When isomorphous substitution happens, sometimes Al+3 slips in so you now have +3 valence and 1 more negative charge (-4+3=-1-). Octahedrons typically have Al+3 in the center but sometimes replaced by +2 so now have excess negative charge because 1 less positive charge. depensing on where and how much subsitutiion happens defines soil properties. considered permanent (not variable, has quantifiable charge) because it is part of the crystal-lattice structure
|
|
Other types of soil colloids: oxide clays
|
Tiny iron particles and aluminum oxides left over after all the silica has leached out of very old soil. common in the tropics. not sticky, no shrink-swell problems. very low amount of ion exchange capacity. were once 2:1 but now only iron oxides and aluminum oxides left (rust particles). this typically happens because of strong acid conditions
|
|
Other types of soil colloids: humus
|
Humus particles are residues of organic matter decay. they are not crystalline, but have irregular, roundish shapes (amorphous). humus is not stable, it decays over time. electrostatic charge is variable depending on pH. humus particles are colloidal and negatively charges. the electrostatic charge of humus is much greater per unit mass than clay. charge due to dissociation of carboxylic (-COOH) and phenolic (-<>-OH) groups. electrostatic charge increases when pH rises (causing dissociation of H in these groups). at high pH, extra OH in solution attracts and pull sof H, leaves (-) charged oxygen. charge increases as pH increases because of dissociation of H
|
|
What determines cation exchange?
|
Cations are attracted to the soil colloids differently depending on: the amount of isomorphous substitution, the location of the substitution within the mineral layers and the type of cation (and its valence). the amount of cation-exchange site bonding depends on: the relative bonding strength of each cation which is based on valence strength (Al3+ > Ca2+ > Mg2+ > K+ > NH4+ > Na+) and the amount of cation present (mass action). substitution is strongest on the surface.
|
|
Cation exchange
|
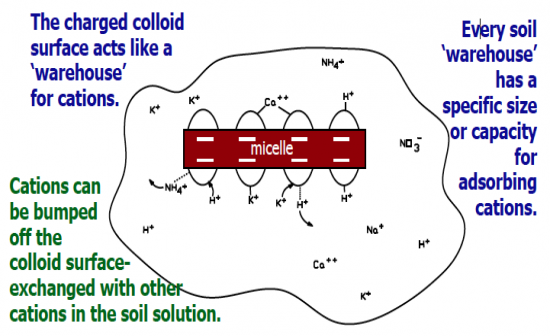 Water can be adsorbed onto surface or absorbed by pores but cations adsorbed on the colloid surfaces are able to change places with other cations in the soil solution. this process is called cation exchange. micelles usually have overwhelming (-) charge. cations are not stuck permanentry and can change depending on what is in the soil water |
|
Mass action
|
The greater the amount of a certain ion in the soil, the more likely it is that this ion dominates the cation exchange sites. can add a bunch of any of these ions and it will change the balance of ions (we do this when we fertilize and lime). flooding to try and restock cations on surface
|
|
Adsorbed ions
|
Are protected from leaching but may be exchanged with other ions in the bulk soil solution. if ions are just dissolves, they are subject to leaching
|
|
Ion exchange
|
Ion exchange occurs when an ion in the bulk soil solution displaces (and REPLACES) an ion that is adsorbed on the colloid surface.
|
|
Catin exchange capacity (CEC)
|
CEC refers to the amount of cations that soil colloids can adsorb for a given soil mass. CEC depends on how much clay is present, and the organic matter (humus) content. CEC is expressed in meq/100g soil. for every 1% of 2:1 clay in a soil's texture, the CEC increases about 0.03-0.5 milliequivalents (meq) per 100g of soil. CEC less than or equal to 10 is low/infertile; CEC greater than or equal to 50 is high. 11 to 20 is moderate and above 20 is high
|
|
CEC comparison
|
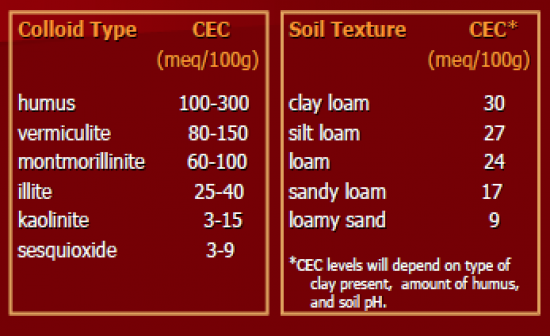 Humus in pure state (don't often find this) can support 100-300meq/100g. sesquioxide = oxide clay in tropical soils. increased clay, increased CEC |
|
Cation behavior at the colloid surface
|
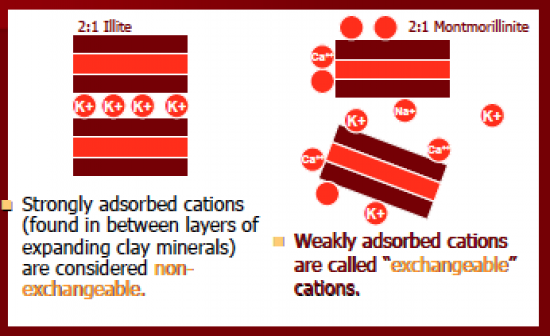 2:1 strongly adsorbed cations (found in between layers of expanding clay minerals) are considered non-exchangeable. non-exchangeable cations are released only when clay weathers and breaks down and becomes different kind of clay (illite weathers to montmorillinite). weakly adsorbed cations are called "exchangeable cations" |
|
Anion storage
|
The exchange of negatively charged soil ions. this is possible when some excess positive charges exist within the clay mineral structure. anion exchange generally is less significant than cation exchange. anion exchange is greatest in acid soils high in oxide clays (tropical oxide clay soils). examples of potentially exchangeable anionic nutrients: NO3-, HPO4(^2-), SO4(^2-), Cl-
|
|
CEC and soil aggregation
|
Clays will tend to disperse when in the presence of monovalent cations. the electrostatic double-layer is diffused and causes micelles to repel one another, clays tend to flocculate (and aggregate) when in the presence of divalent (or polyvalent) ions, where the ionic double layer is compressed. this dispersion is why Na+ is bad in soil (no flocculation). add something like Ca+2 to get soils to aggregate
|



