Cards In This Set
| Front | Back |
|
Using the periodic table identify:
|
 |
|
How do you know how many protons are in an element?
|
The atomic number indicates the number of protons.
|
|
How many neutrons does an element have?
|
The atomic mass for an element is the sum of both protons and neutrons.
|
|
How many electrons does an element have?
|
The number of electrons equals the number of protons.
|
|
What is the ionic charge?
|
When neutral atoms collide, a negative electron is transferred from one atom to another, and both atoms become particles called ions, which have an electrical charge. If an atom has lost electrons the overall charge becomes positive and if it gains electrons the overall charge is negative.
|
|
Example for Oxygen (O).
|
Atomic number: 8
Number of protons: 8 Number of electrons: 8 Atomic Mass: 16.0 Number of neutrons: atomic mass - number of protons = 16.0 - 8 = 8 Ionic charge: 2- (means oxygen has gained two electrons) |
|
Bohr model for oxygen.
|
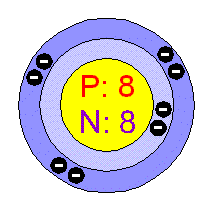 |
|
Referring to the Bohr model for oxygen, how many energy levels are there?
|
2 energy levels. 8 electrons altogether
First level has 2 atoms (2n²) Second level has 6 atoms |
|
Distinguish between ionic and molecular compounds.
|
Ionic Compounds"
- bonds are created by the transfer of electrons - high melting point - distinct crystal shape - formed from metallic and non-metallic elements - forms ions in solution - conducts electricity - solid at room temperature Moleculary Compounds - bonds are created by the sharing of electron - low melting point - not always form crystals - usually formed from only non-metallic elements - does not form ions in solution - usually does not conduct electricity - solid, liquid or gas at room temperature |
|
What are diatomic molecular compounds?
|
Molecules that are made of two atoms of the same element.
For example: iodine (I2), hydrongen (H2), nitrogen (N2) |
|
What are the rules for naming an ionic compound?
|
1. The name includes both elements in the compound, with the name of the metallic element first.
2. The non-metallic element is second. Its ending is changed to -ide Example: CaCl2 1. calcium (M) and chlorine (NM) 2. calcium chloride |
|
What are the rules for naming molecular compounds?
|
1. Write the entire name of the first element
2. Change the ending of the second element to -ide 3. Use a prefix to indicate the number of each type of element in the formula mono = 1 di =2 tri = 3 tetra = 4 penta = 5 Mono is only used for the second element as in carbon monoxide Example: CCl4 1. carbon 2. chlorine --> chloride 3. carbon tetrachloride |
|
What does H2O mean?
|
2 atoms of hydrogen and 1 atom of oxygen
|
|
How is the state of matter written?
|
CH4(g)
(g) means it is in the gas state (s) means it is in the solid state (l) means it is in the liquid state (aq) means it is dissolved in water |
|
What is an ion?
|
An atom or group of atoms that has become electrically charged
|



