Cards In This Set
| Front | Back |
|
13.1.1: Describe
the photoelectric effect.
|
The phenomenon
where electrons are emitted from the surface of a metal after being exposed to
light of a particular frequency called the threshold frequency. Light below the
threshold frequency will not cause electrons to be emitted. Above the threshold
frequency, the maximum KE of the electrons depends on the frequency of the
incident light. The number of electrons emitted depends on the intensity of
light, and not the frequency. Emission of electrons is instantaneous, and
considers that light can act as particles called photons.
|
|
13.1.2: Describe
the concept of the photon, and use it to explain the photoelectric effect.
|
Students should be able to explain why the
wave model of light is unable to account for the
photoelectric effect, and be able to describe and
explain the Einstein model.
Light travels in discrete, packets of energy called photons. The energy of the photon is given by the equation E=hf, where h is Planck’s constant and f is the frequency of the light. The photon explains the photoelectric effect because it shows that light can act as particle and not just a continuous wave of energy. If light traveled solely as a wave, any frequency of light would cause an emission of electrons. This is not the case. The threshold frequency exists, below which no electrons are emitted. Ephoton = Eelectron + Ekinetic hf =φ+Ek hf = hf0 + Ek Ek(max) =hf −φ |
|
13.1.3: Describe
and explain an experiment to test the Einstein model.
|
Millikan’s experiment involving the application of a
stopping potential would be suitable.
The Stopping Potential Experiment is used to answer this objective. A simple circuit consisting of an evacuated chamber, voltmeter, ammeter, and variable voltage supply was used to determine the maximum KE of the emitted electrons for various frequencies of light. The evacuated chamber consisted of two electrodes (anode and cathode or positive and negative conductors). One electrode was exposed to incident light. If the light was above the threshold frequency for the metal electrode, a current registered in the ammeter. The voltage was reversed so that the electrons causing the current would be repelled. The voltage that reduced the current to zero, also called the stopping voltage can be used to calculate the maximum KE of the emitted electrons, Regardless of the intensity of the light incident on the metal (which increases the current), the stopping voltage is always the same for a given frequency. E =hf −φ Vsq=hf −φ |
|
13.1.5: Describe the de Broglie hypothesis and the concept of matter waves.
|
Students should also be aware of wave–particle
duality (the dual nature of both radiation and
matter).
The de Broglie hypothesis states that all particles can act and travel as waves and have an associated wavelength defined λ=p/h, where p is the momentum of the particle, and h is Planck’s constant. Matter waves are the waves associated with particles or matter. They have an associated de Broglie wavelength. |
|
13.1.6: Outline an experiment to verify the de Broglie hypothesis.
|
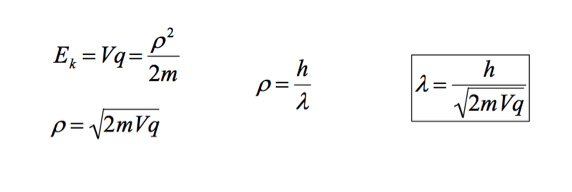 A brief outline of the Davisson–Germer experiment will suffice. One of the experiments used to verify the de Broglie Hypothesis was the Davisson and Germer electron diffraction experiment (not necessary to know name). Davisson and Germer used an electron diffraction pattern to determine the wavelength of an electron. The experimental wavelength corresponded to de Broglie’s calculated wavelength. The wavelength of an electron can be calculated using the de Broglie hypothesis and accelerating an election through a potential difference. The KE of the electron is equivalent to Vq. (KE = Vq). |
|
13.1.8: Outline a laboratory procedure for producing and observing atomic
spectra.
|
Students should be able to outline procedures for
both emission and absorption spectra. Details of
the spectrometer are not required. Emission Spectra — Atomic spectra can be observed by exposing an atom of
an element to a continuous source of energy.
Examples of laboratory procedures used to produce and observe atomic
spectra include the “flame” test and exposing spectral tubes to high voltage
Absorption Spectra — View light shining through a sample (usually a gas)
with a spectroscope.
|
|
13.1.9: Explain how atomic spectra provide evidence for the quantization of
energy in atoms.
|
An explanation in terms of energy differences
between allowed electron energy states is
sufficient. Max Planck suggested that electrons change their energy in quantized
amounts (E=hf). Spectral lines show that electrons shift orbits releasing or
absorbing discrete amounts of energy. Electrons occupy discrete energy levels.
Movement between levels involves absorbing or emitting energy. When an electron
moves from a higher energy level to a lower energy level, it emits energy in
the form of light (photons).
|
|
13.1.11: Explain the origin of atomic energy levels in terms of the “electron in
a box” model.
|
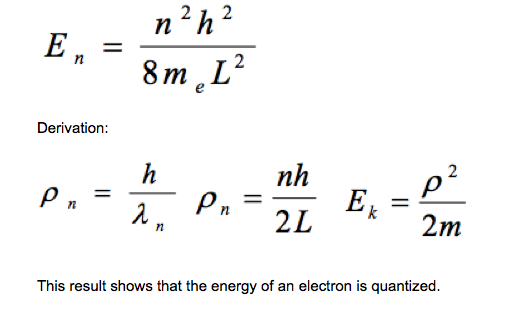 The model assumes that, if an electron is confined to move in one dimension by a box, the de Broglie waves associated with the electron will be standing waves of wavelength 2L/n where L is the length of the box and n is a positive integer. Students should be able to show that the kinetic energy EK of the electron in the box is given by (see image): |
|
13.1.12: Outline the Schrödinger model of the hydrogen atom.
|
The model assumes that electrons in the atom
may be described by wavefunctions. The electron
has an undefined position, but the square of
the amplitude of the wavefunction gives the probability of finding the electron at a particular point. Electrons orbit in a probability region or electron cloud. The solution to the equation predicts exactly the line spectra of the hydrogen atom. |
|
13.1.13: Outline the Heisenberg uncertainty principle with regard to
position–momentum and time–energy.
|
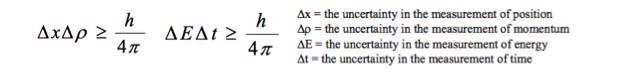 Students should be aware that the conjugate quantities, position–momentum and time–energy, cannot be known precisely at the same time. They should know of the link between the uncertainty principle and the de Broglie hypothesis. For example, students should know that, if a particle has a uniquely defined de Broglie wavelength, then its momentum is known precisely but all knowledge of its position is lost. |
|
13.2.1: Explain how the radii of nuclei may be estimated from charged particle
scattering experiments.
|
One
experiment that can be used is the Geiger Marsden experiment. An estimate of
the radii of nuclei can come about through the principle of the conservation of
energy. The KE of a particle approaching a nucleus is completely transformed
into electrostatic potential energy. At the distance of closest approach, the
KE will be converted entirely to PE. The particle will be momentarily at rest.Use of energy conservation for determining
closest-approach distances for Coulomb scattering
experiments is sufficient.
|
|
13.2.2: Describe how the masses of nuclei may be determined using a Bainbridge
mass spectrometer.
|
Students
should be able to draw a schematic diagram of the Bainbridge mass spectrometer,
but the experimental details are not required.
Students
should appreciate that nuclear mass values provide evidence for the existence
of isotopes.
Positive
ions of elements are pass through / accelerated through electric and magnetic
fields. Particles will pass into a detector. The mass of the nuclei can then be
determined.
eE = Bev
or v = E/B
|
|
13.2.3: Describe one piece of evidence for the existence of nuclear energy
levels.
|
For example,
alpha (α) particles produced by the decay of a nucleus have discrete energies;
gamma-ray (γ-ray) spectra are discrete.
Students
should appreciate that the nucleus, like the atom, is a quantum system and, as
such, has discrete energy levels.
When a nucleus decays by emitting an alpha particle
or a gamma ray, the particles or photons emitted are only at specific energies
(there is not a complete range of energies emitted, only certain specific
levels).
An alpha particle or photon thus has an energy equal
to the difference between energy levels of the nucleus.
The gamma and alpha particle energies are
characteristic of the emitting nucleus. The existence of nuclear energy levels
is proven.
|
|
13.2.4: Describe β+ decay, including the existence of the neutrino.
|
Students should know that β energy spectra are
continuous, and that the neutrino was postulated
to account for these spectra.
|
|
13.2.5: State the radioactive decay law as an exponential function and define
the decay constant.
|
Students
should know that the decay constant is defined as the probability of decay of a
nucleus per unit time.
If the number of nuclei present in a sample at t = 0
is N0, the number N still present at time t later is given by
N = Noe-λt
where λ is the decay constant (the probability that a
nucleus will decay in unit time).
|



