Cards In This Set
| Front | Back |
|
What is organic chemistry?
|
The study of compounds of carbon.
Organic compounds are made up of carbon and only a few other elements, mainly hydrogen, oxygen and nitrogen COHN but also, sulfure, phosphorus and halogens (flurine, chlorine, bromine or iodine) |
|
What are halogens?
|
Anything in Group 17
e.g. Fluorine, Chlorine, Bromine, Iodine |
|
Whay is organic chemistry a separate disipline within chemistry?
|
Scientists at one time believed that a "votal force" present in living organics was necessary to produce an organic compound.
|
|
Who was the scientist and what year brought the demise of the "vital force" of organic chemistry?
And the equation. |
The experiment of Wohler in 1828 was th first in a series of experiments that led to the demise of the vital force theory.
NH4Cl + AgNCO -heat-> H2N-C=O-NH2 + AgCl Ammonium chloride + silver cyanate -> Urea + silver chloride |
|
How many organic compounds are there?
|
Chemists gave discovered or made over 10 million organic compounds and an estimated 100,000 new ones are discovered or made every year.
By comparison, chemists have discovered or made 1.7 million inorganic compounds. Thus - approximatly 85% of all known compounds are organic |
|
What is the link to biochemistry?
|
Carbohydrates, lipids, proteins, enzymes; nucleic acids, hormones, vitamins and almost all other chemicals in a living system are organic compounds.
|
|
What are the difference between organic and inorganic compounds. (Table)
|
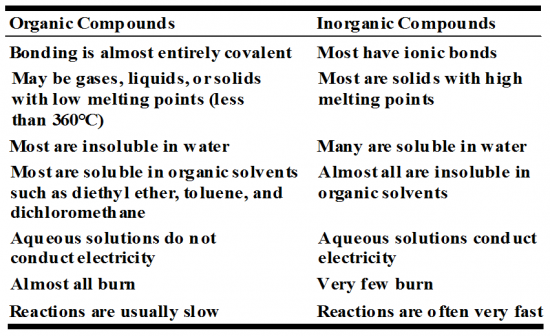 |
|
What type of bonds do organic / inorganic compounds have?
|
Organic = almost entirely covalent
Inorganic = most have ionic bonds |
|
What state are organic / inorganic compounds usually?
|
Organic = solid, liquid, or gas with low melting points (less than 360oC)
Inorganic = Most are solid with high melting points |
|
Are organic / inorganic compounds soluble in water?
|
Organics = most are insoluble, non-polar molecules and those with a carbon chanin longer than 5 (pent-) then it is so large the polar portion does not have as greater attraction force.
Inorganic = may a soluble in water as they are ionic compounds (SPAAN) and have a differently charge atoms (compounds). |
|
Are organic / inorganic compounds soluble in organic solvents? and what is an example of a solvent?
|
Organic = most are soluble in organic solvents
Inorganic = most are insoluble in organic solvents e.g. diethylether, toluene, dichloromethane |
|
Do organic / inorganic compounds conduct electricity?
|
Organic = aquesous solutions do not conduct electricity
Inorganic = aquesous solutions conduct electricity |
|
Do organic / inorganic compounds burn?
|
Organic = Almost all burn
Inorganic = Very few burn |
|
What is the speed of a reaction in organic / inorganic compounds?
|
Organic = usually slow
Inorganic = reactions are often fast |
|
Draw the VSEPER / Structural model of Ethane, Ethene Acetylene, Chlororethane, Methanol, Formaldehyde, Methanamine, Methyleneimine
|
 |



