Cards In This Set
| Front | Back |
|
ABSOLUTE ZERO
|
THE COLDEST TEMP. THERE IS... -273 C cant be reached... all atomic energy stops
|
|
Actual yield of a chemical reaction
|
The quantity of a product obtained from a chemical reaction.
|
|
Atmosphere
|
Unit of measurement: 760mm (pressure)
|
|
Atomic size
|
On the periodic chart: increases from top to bottom, and decreases from left to right
|
|
Avogadro's law
|
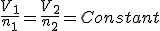 |
|
Balanced chemical equation
|
An equation in which the number of each type of atom on both sides of the equation is equal.
|
|
Barometer
|
An instrument used to measure pressure. (mm hg)mm of mercury
|
|
2 types of bent structures
|
Trigonal planer, tetrahedral. both have unshared pair.
|
|
Bohr atomic model
|
1s2, 2s2,3s2,3p6.... example of bohr model (quantum mechanical model)
|
|
Bonding pair
|
Pair of electrons that bond between two atoms (lewis dot diagram)
|
|
Bonding theory
|
Theory that predicts how atoms bond together to form molecules.
|
|
Boyles law
|
P1V1=P2V2 (temp and mol. constant)
|
|
CHARLES LAW
|
V1 = V2T1 T2
|
|
Chemical bond
|
The sharing or transfer of electrons to attain stable electron configuration for the bonding atoms.
|
|
Chemical equation
|
Chemical reactions are represented by chemical equations ex: CH4 + O2--->CO2 + H2O
|



