Cards In This Set
| Front | Back |
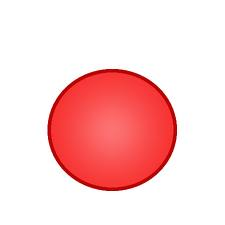 Which scientist had this model of an atom? |
Dalton
|
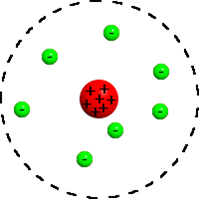 Which scientist had this model of an atom? |
Rutherford
|
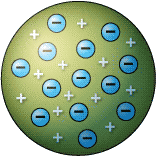 Which scientist had this model of an atom? |
Thomson
|
 Which scientist had this model of an atom? |
Schrodinger
|
 Which scientist had this model of an atom? |
Bohr
|
 What was Dalton's main discovery? |
All matter is made out of atoms.
Atoms of one element are identical.
|
 What was Thomson's main discovery with his Plum Pudding model? |
The atom is made out of two parts:
the negative ELECTRON and a postive piece
|
 What were Rutherford's two main discoveries? |
1-The atom is mostly empty space
2-There is a positively charged NUCLEUS
|
 What wasBohr's main discovery with his planetary model? |
The negative electrons move in orbits around the nucleus
|
 How is Bohr's model of the atom different from the one we draw today? |
We draw NEUTRONS in the nucleus
Bohr did not know about this when he made his model. He thought the nucleus had only positive particles.
|
|
What was Chadwick's main discovery?
|
The NEUTRON
|
|
What is the current model of the atom?
|
 The electron cloud model |
|
In which model do the electron move rapidly around the atom (not on orbits or rings)?
|
 The electron cloud model |
|
Which part of the atom has a POSITIVE charge?
|
The Positive
PROTON
|
|
Which part of the atom has a NEGATIVE charge?
|
The negative
ELECTRON
|



