Cards In This Set
| Front | Back |
|
What Is an Atom?(What is an Atom made from & Where are the particles found in an Atom)
|
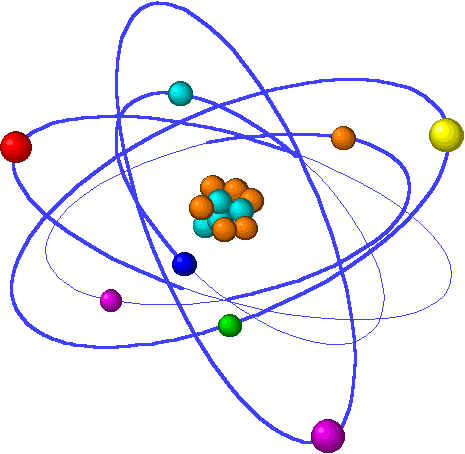 The Basic Unit of matter |
|
What is an Isotope?
|
Atoms of the same element that differ in the number of Neutrons they contain.
|
|
What is an Ion?
|
Positively and Negatively Charged ions.(lost a Atom or gained an atom:lost a electron or gained an electron)
|
|
What is a Convalent bond?
|
Form when atoms share electrons.
|
|
What is an Ionic Bond?
|
Formed when one or more electrons are transferred from one atom to another.
|
|
What are Van Der Waals forces?
|
A slight attraction between oppositely charged Molecules.
|
|
What is a Chemical Formula?
|
A formula used to describe the types of atoms and their numbers in an element or compound.
|
|
What is a Chemical Reaction?
|
A process in which one or more substances are changed into a new substance.
|
|
What are the Parts to a Chemical Reaction?
|
Reactant: a substance which is in a chemical reaction.
Product: substance which is produced by the chemical reaction.
|
|
Why are Water Molecules Polar?
|
 Because of it's shape. |
|
What is the definition of Suspension?
|
Mixture of water and non-dissolved materials.
|
|
What are Acids?
|
Compound that forms hydrogen ions (H+) in solution.
|
|
What are Bases?
|
Compound that produces hydroxide ions (OH+) in Solution.
|
|
A) Does an Acid or a Base have a higher concentration of H+ ions? b) Where are they found on a pH scale?
|
A) Acids have higher concentrations of H+ ions.b) Acids on lower numbers (under 7) / bases on higher numbers (over 7)
|
|
What is the main organic source of energy for living things?
|
 The Sun |



